From product development to quality control
The “Made Smarter Innovation – Digital Medicines Manufacturing Research Centre“ (DM2) project was cocreated and is co-delivered by industry partners – pharmaceutical companies and technology suppliers such as MEDELPHARM as well as CMAC (Centre for Continuous Manufacturing and Advanced Crystallization) at the University of Strathclyde. This project is delivered across five platforms with one of them delivering a “Self-Driving Tableting DataFactory”. This platform pursues the goal of accelerating the development of pharmaceutical products and quality control strategies using industrial digital technologies – integrating artificial intelligence (AI), robotics and digital twins. The research project is publicly funded and required a substantial co-investment from industry (see box on p. 11). The platform is led by Dr Daniel Markl, Associate Director of CMAC.
Proprietary data situation
The path to the transformation of the development of tablets begins with a distinctive feature of the pharmaceutical industry: “We lack structured, cross-company data; other sectors have millions of them for AI applications, we only have hundreds,” says Markl, explaining the point of departure. As a result, there are hardly any negative test results and failures, which are essential for creating predictive data-driven models. In addition, much of the data is in a proprietary format so that it cannot be accessed. To unlock the potential of emerging AI technologies, the sector requires a standardized data structure – across all instruments and processes.
CMAC’s Quality by Digital Design approach
On the one hand, the processes themselves follow the research trend for self-driving laboratories. On the other hand, digital tools such as “AI”, “collaborative robotics” and “real-time optimization” complement the scientific principle of QbD (Quality by Design), leading to a Quality by Digital Design (QbDD) approach as developed at CMAC. This allows the team to establish an automated “design – make – test – analyze” workflow: using the formulation process as an example, data-driven models design the experimental conditions, the automated tableting system makes the tablets under these conditions, tests them and analyzes the data automatically to update models and identify the next experiments.
This approach creates a “multidimensional, model-based parameter space”, explains Markl, which “significantly reduces the number of experiments used to develop a product and increases the probability of an optimal process.” Nevertheless, this approach does not completely replace experiments, as they are still essential for compliance with pharmaceutical industry regulations: “We use models to identify the experiments with the highest probability of providing maximum information,” explains the scientist. The challenge industrial development poses is that the decisions made in the early stages are based on small quantities of material, but “have a major impact on later changes.” Markl therefore welcomes the fact that industry‘s attitude towards connectivity and data sharing is changing: “Everyone now recognizes the value of cross-company data.”
For Markl, the processes with the regulatory authorities are the “biggest obstacles”. The use of AI and data-driven modeling is viewed critically. “We need to make the technologies more transparent by working together across the sector in a pre-competitive manner,” the scientist demands. Developing the authorities’ competence in these emerging technologies is also important. The US Food and Drug Administration (FDA), for example, is already working closely with academia and industry, investing in regulatory science and innovation into these topics.
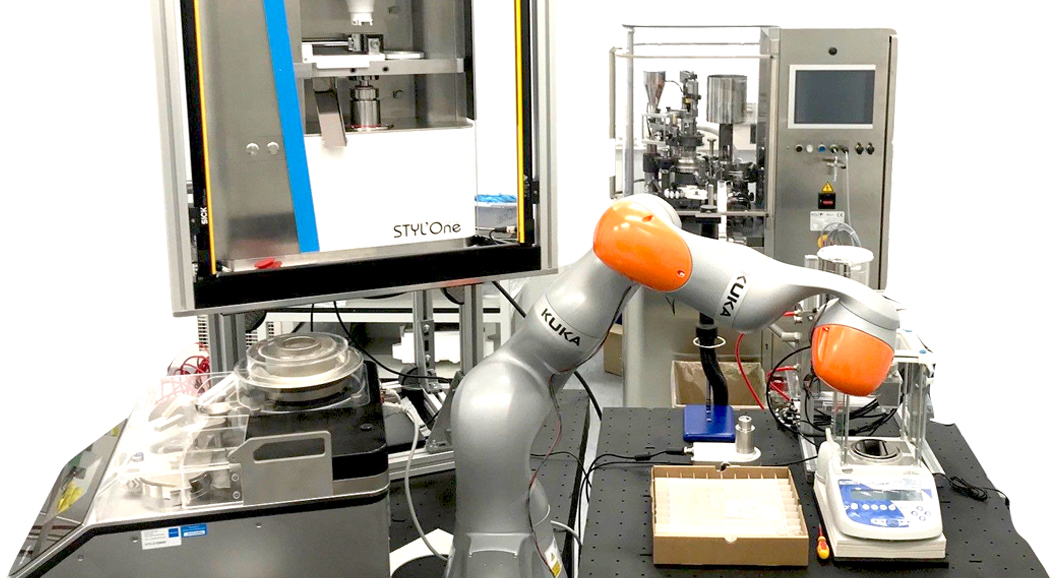
Suitable for all steps: the STYL‘One Nano
The companies involved are supporting the project through in-kind contributions and financially, but “their commitment is crucial in every respect,” explains Markl. KORSCH and MEDELPHARM‘s keen interest in research, combined with the use of the STYL‘One Nano compaction simulator, was a decisive factor in the development of the project: on the one hand, its precise punch displacement control and advanced instrumentation in a compact benchtop design provide the essential prerequisites for automated processes. On the other hand, its flexibility unlocks new research, as Markl reports: “The system was optimized for our needs in close collaboration with Quentin Boulay from MEDELPHARM. For example, the powder can now be moved automatically into the compaction simulator with a robotic arm and the compaction simulator can be fully controlled externally.”
In this respect, the STYL‘One Nano is “a perfect solution for us,” the expert summarizes. It is suitable for future laboratory environments, even if its digital interface is already currently user-defined: “Every device in our lab should have a standardized digital interface in the future,” explains Markl. This will enable the seamless digital integration of instruments and processes to send signals, determine parameters, start processes, and collect structured data. Markl hopes that standard interfaces with standard protocols will be developed to accelerate the adoption of industrial digital technologies in the industry, and ultimatively bringing new medicines to patients faster.
The KORSCH XP 1 single-punch tablet press is also being used by the scientific community in Strathclyde, generating hundreds of data points a week in several pre-competitive industrial projects at CMAC. Furthermore, many of the PhD students at the Centre will be using it extensively for their research projects over the next years, so the university will continue to have a need for the KORSCH and MEDELPHARM instruments.
Centre for Continuous Manufacturing and Advanced Crystallisation (CMAC)
CMAC, based at the University of Strathclyde in Glasgow, UK, is an internationally leading manufacturing research centre with a unique configuration of academic research, applied, and pre-competitive programmes. Working in partnership with Tier 1, Tier 2, academic, and innovation partners, CMAC‘s goal is to transform Medicines Development, Manufacture & Supply, and further grow its world-class portfolio of multi-disciplinary collaborative research. This includes the Digital Medicines Manufacturing Research Centre (DM2), Digital Design and Manufacture of Amorphous Pharmaceuticals (DDMAP), UKRPIF Net Zero Pilot, UKRPIF CMAC Data Lab, and the CMAC Future Manufacturing Research Hub, along with training and translation projects within a world-class facility.
The Digital Medicines Manufacturing (DM2) project
The state-of-the-art facility CMAC is home to the DM2 (Digital Medicines Manufacturing) project, a 3.5 year project that started in October 2022 in collaboration with MEDELPHARM and many other industry partners. With the focus on enabling digital design, DM2 is a flagship programme for delivering CMAC’s strategic priorities to improve digital maturity in medicines manufacturing and develop the workforce of the future who can flourish in these digitalised lab environments. DM2 is co-funded by the Made Smarter Innovation challenge at UK Research and Innovation (UKRI) and industry investments. Contact persons at MEDELPHARM are Quentin Boulay, Product Marketing Manager, and Bruno Leclercq, Global Scientific Coordinator.
About Daniel Markl
Daniel Markl is Professor in Pharmaceutical Product Engineering at the University of Strathclyde, UK, and Associate Director at CMAC since 2018. He leads Platform 2 (Autonomous Microscale Manufacuturing) within the DM2 project. He holds a PhD in Chemical Engineering from TU Graz, Austria and was a Research Associate at the University of Cambridge, UK, before joining CMAC.